Your Cart is Empty
Free Shipping on orders over $75!
Study name: Do portable recreational oxygen supplements share the same physiological benefits as other forms of hyperoxic environments during moderate intensity cycling? (The Oxygen Plus Cycling Study)
Gender/sex and ability: Seven healthy young, male middle and long-distance runners underwent a repeated measures design
Type of exercise used: Cycling
Intensity of exercise: Five sets lasting 30 seconds each at 80% maximum intensity
Percent (%) of oxygen used: 21% or 99.5%
Research/study findings: During the visit involving the inhalation of a personal oxygen supplement, participants blood lactate levels had decreased by 24% post exercise, when compared to the same trial inhaling normal air. The study found physical improvements during exercise, as a result of the inhalation at increased oxygen levels provided by Oxygen Plus. This is the first study to find a significant result whilst supplementing with this form of O2 inhalation and confirms physiological benefit when the product is used with exercise. The physical improvements included reductions in lactic acid (also known as lactate) after exercise, by up to 24%, when using Oxygen Plus immediately before, during and after exercise. The reduction of lactic acid can postpone muscle fatigue mechanisms and prolong endurance when used during exercise, as well as, speeding up recovery between bouts of exercise, as lactic acid is processed at a greater efficiency. Study highlights Oxygen Plus is an optimum method of increasing oxygen to individuals in a portable and easily accessible way.
Oxygen Plus application/implication: Healthy people who breathe Oxygen Plus during and after short-term, moderate intensity exercise can increase their endurance and speed up recovery. Exercise enthusiast and athletes can improve their performance through a reduction in lactic acid within the blood, which leads to faster recovery (increases in recovery speed) and endurance (prolonged physical activity).
Study excerpts:
Publication/source/year: Wyton, Lee (2018). Do portable recreational oxygen supplements share the same physiological benefits as other forms of hyperoxic environments during moderate intensity cycling?, University of Chichester, Sport and Exercise Department.
The full study is provided below. Contact Oxygen Plus for more information on the study.
© 2020 Oxygen Plus, Inc. All Rights Reserved.
Abstract
The purpose of this study was to investigate the effects of hyperoxia in the form of portable recreational oxygen (O2) during a moderate intensity cycling performance. To determine whether this form of supplement provides physiological benefits to recovery limitations found from during physical activity when compared to normoxic conditions. Participants (n=7) underwent a repeated measures design, having been free from previous respiratory and musculoskeletal conditions. The first cycling visit (Visit 1) established participant’s 80% maximal intensity (Watt), followed by two additional visits containing 5 x 30 second efforts with 90 seconds recovery before beginning the next effort. Visit 2 introduced the hyperoxia supplement, which was self-administered. The third visit (Visit 3) occurred one week later with the same efforts, however, under conditions of normoxia. Five breaths of the supplement were required prior to and after each of the 5 efforts, as well as post cycling. Duration of one breath was defined as inhaling as deeply and continuously as possible. Heart rate (HR), O2saturation (SpO2) and rate of perceived exertion (RPE) were obtained before and after each of the 5 efforts, as well as post exercise. Blood lactate was collected pre and post cycling. Data was analysed under a pair sample t-test if normally distributed. If not normally distributed, a Wilcoxon signed rank test was used. HR and SpO2was found to be non-significant (p > 0.05) between hyperoxia vs. normoxic conditions. Post exercise hyperoxia and normoxic for blood lactate was found to be significant (p < 0.04), having a 29% difference in blood lactate between the two visits. This latter finding suggests that the portable recreational O2 used in this study under similar conditions can promote recovery and endurance in short term moderate cycling by decreasing blood lactate.
Introduction
The ability to prolong physical activity is the focus ofathletes and recreational enthusiasts in all sport and exercise disciplines. From rest, maintaining or increasing physical demand provokes our cardiac output to oxygenate blood rapidly (Joyner & Coyle, 2008) and is achieved by incorporating the musculoskeletal, respiratory and cardiovascular systems (Kim & Greenburg, 2013). Through these physiological systems, the ultimate result is to transport an increase in total blood to skeletal muscle where it can extract oxygen (O2) from haemoglobin to continue producing ATP (Bassett & Howley, 2000), a process undergone through the electron transport chain (Mougios, 2006; Plowman & Smith, 2013). A simple solution to increase O2 delivery is through external sources, in the form of hyperoxic mixtures. Hyperoxia is the excess supply of O2 at higher concentrations of normoxic air, which at sea level remains at 20.95% (Mach, Thimmesch, Pierce & Pierce, 2011). Research has found that cardiac output and stroke volume decreased in demand when inhaling O2 at an increased percentage from baseline level, signifying the ability to prolong engagement in activity (Haque et al., 1996; Young, 2012). Additional physiological influences on exericse parameters have been found through inhaling increasedO2include 1) areduction in blood lactate concentration (Stellingwerff et al., 2005; Stellingwerff et al., 2006; Brinkmann, Bloch & Brixius, 2018) and, 2) a restoration in O2 saturation (SpO2) (Dempsey, Hanson & Henderson, 1984; Sperlich, Zinner, Hauser, Holmberg & Wegrzyk, 2017).
From a practical point of view, hyperoxia administered before, during and immediately after exercise can provide benefits in varying physical activities (Mallette, Stewart & Cheung, 2017). Examples include individual activities or team sports involving running, swimming and/or cycling, in which O2supplementation benefits in three key ways: 1) improved physical performance from prolonged physical endurance (Sperlich et al., 2011; Yokoi et al., 2014); 2) quicker recovery in-between efforts/sets of exercise (Oussaidene et al., 2013; White et al., 2013); and, 3) physiological enhancement on the effects of training – such as increasing cycling power or running speed (Bangsbo et al., 1994; Burton, Stokes & Hall, 2004; Segizbaeva & Aleksandrova, 2009; Boushel et al., 2014). Traditionally, administering hyperoxic O2 requires special equipment such as large tanks with facial masks or environments that are confined to chambers that increase O2 within that area. The issue presented when using these methods is that O2supplementation cannot be easily administered during exercise due to logistics and cost barriers (White et al., 2013; Hauser et al., 2014). Additionally, ample supply of O2has not been easily obtainable for recreational use, due to high costs and lack of accessibility through sales outlets. Because of these challenges of inaccessibility, cost and availability, companies have recently created a new form of O2 supplements that incorporates more accessible methods of administering hyperoxia to a wider population, in the form of portable O2 canisters. For the past four years, the World Anti-Doping Agency (WADA) does not consider the use of recreational O2 products (hyperoxia), nor its components, a manipulation of the blood within competitive sport (WADA, 2018).
Literature on portable O2 supplements is limited and its effects on physiological responses during exercise is currently not as established when compared to O2 tanks or chambers. Etheredge, Judge and Bellar (2014) produced the first study to explore portable recreational O2 supplements and their influence on physical performance during moderate intensity exercise that exhausted participants. They concluded these supplements withheld no significant effect to participant’s performance during and post running relating to time to exhaustion, respiratory rate, carbon dioxide (CO2) clearance and reductions in heart rate (HR) reduction or rate of perceived exertion (RPE) when compared to a placebo (normal air); however, this study did identify limitations within its methodology. Fundamentally, the O2 supplement was administered through a tube leading into the participant’s mouth/airway, which is not an optimum form of application as instructed by manufacturers. They advise these supplements to be administered directly into the path of inhalation at the mouth and/or nose. Because of this, the O2concentration within the canister (> 90%) became contaminated with normoxic air as it passed through the tube to the participants airway, thus only increasing inspired O2 (FiO2) by 1.5%. It can be presented that no physiological benefits were found due to inhaling a FiO2at only 22.4%, opposed to the minimal30-99% found in previous literature.
Therefore, the aim of this study was to examine whether inhaling hyperoxia in the form of portable recreational O2 canisters during exercise can produce physiological benefits on short term, moderate intensity cycling. It was our hypothesis that these types of supplements would have some physiological benefit towards exercise.
Methods
Sample
Seven male sport students of the University of Chichester volunteered for the study. A completed health history questionnaire and consent form were required pre-testing. Participants were excluded from the study if they withheld any musculoskeletal injuries or respiratory disorders. Suitable clothing for exercise was requested prior to arriving, along with abstaining from consumption of caffeinated drinks and strenuous exercise 12 hours prior to testing.
Table 1 displays participants (n = 7) demographic data – age (years), mass (kg), height (meters) and body mass index (BMI) in the format of mean ± stand deviation (SD)
|
Demographics |
Mean ± SD |
|
Age |
23.3 ± 2.0 |
|
Mass |
79.1 ± 22.9 |
|
Height |
176.0 ± 7.7 |
|
*BMI |
23 ± 9 |
*BMI: Shown as medium, IQR.
Maximal intensity effort
The first visit (Visit 1) included collection of participant’s demographic measurements such as height, age, mass and sex (Table 1). BMI was then calculated using participants mass and height, with the equation of weight (kg) divided by height meters squared. Physical testing was conducted on a cyclometer (Excalibur Sport 925900 (upright), Lode, Groningen, Netherlands), with seat height set to include a knee bend at flexion of 25-30°, to minimise knee injury (Bini, Hume & Croft, 2011). The maximal test was in the form of an incremental stepwise test, beginning intensities for males starting at 75Watts (W) and increasing by 15W per minute until participants could no longer continue. HR and RPE was collected at baseline and at the beginning of each stage, maximal W intensity was collected post effort (Table 2). The final W was then calculated to 80% of the total number, which was then the intensity used for visit 2 and visit 3.
Table 2 displays participant’s maximal intensity data at the end of visit 1 for HR, Wand RPE
|
Physiological measurements |
Mean ± SD |
|
HR (baseline) |
81.1 ± 8.23 |
|
HR (final) |
176.5 ± 7.9 |
|
W |
270 ± 65.4 |
|
*RPE |
18, 2 |
*RPE: Shown as medium, IQR
Experimental approach for the aim
The study had a repeated measures design, with participants required to undertake three testing visits in a consecutive weekly fashion. Visit 2 and visit 3 include a brief demonstration of the test procedure, followed by participants cycling for 30 seconds at 80% of their maximum intensity gained in visit 1. A passive recovery of 90 seconds before cycling another 30 second was required, which was repeated until participant completed all five (5) efforts of 30 second (5x30s) cycling efforts. All participants were advised to maintain cycling cadence at 70 RPM, and a self-selected warm up of ~5 minutes was carried out prior to the main testing. If participants felt sick or dizzy during any testing they were informed to stop immediately. On visit 2, the hyperoxia supplement was self-administered in participant’s own time, prior to beginning the first, and after each of the 5x30s cycling efforts. The third visit contained normoxic conditions, in which no supplement was administered and normal (FiO2 = 20.95%) air was inhaled. HR (b .min-1), RPE (Borg, 1982) and SpO2 was measured at baseline (before administering supplement), after each cycling effort and post effort. Blood lactate(mmol/L) was collected using finger lancing techniques preceding and after all 5 efforts had been completed. Blood lactate collections were analysed using a lactate analyser (YSI 2300 STAT Plus Glucose Lactate Analyzer). These samples were collected from participant before any cycling had begun (including warm up) and as soon as cycling had concluded < 60 seconds.
Portable Oxygen Supplement
Chichester (UK) is located 20 meters (66 feet) above sea level, therefore contains 20.95% of O2 within the surrounding atmosphere. To increase this volume of O2, a hyperoxia supplement (Oxygen Plus, Inc. Minnesota, USA) was used, containing 3.420ls of 97% (± 2%) pure O2 in a portable, lightweight canister that dispenses O2, and is inhaled, when the actuating button is depressed. These supplements were self-administered directly into participant’s nasal pathway for five full inhalations, with instructions to inhale as much and as deeply as possible, holding the trigger down firmly with their finger or thumb of either hand. The ability and duration of inhalation to a point deemed at lungs full capacity varied between participants. The nasal pathway for inhalation of the supplement was chosen as a safer method than oral, as this reduces the risk of carbon dioxide rebreathing (O'driscoll, Howard, Earis & Mak, 2017). The brand and design of this portable recreational O2 canister expels hyperoxic air for as long as the trigger is pressed, with light or heavy pressure on the trigger having no effect on the quantity expelled. After ~15 to 25 breaths were taken during visit 2, the air flow expelling from the canister decreased in velocity, an occurrence that was heard and felt. When this lower flow rate occurred, it was at the discretion of the researcher to replace the canister with a new, full canister, in order to maintain maximum effects of the supplement. The method of administering the O2 followed the guidelines of the manufacturer (Oxygen Plus, Inc.), which was noted on the canister.
Statistical analysis
The collection of raw data was transferred into IBM SPSS, version 23 – software for Windows(SPSS, Inc. Chicago, IL, USA). Normality was assessed prior to any inferential statistics withskewness and kurtosis (cut-off ±1.96) along with a Kolmogorov-Smirnov test used to detail non-significance (p > 0.05). Data was found to remain within the selected cut off and non-significant, other than SpO2 during the hyperoxia visit and the pre-blood lactate hyperoxia visit. Central tendency was measured in our quantitative data, HR, blood lactate and SpO2deemed into means and standard deviations (SD) (mean ± SD); RPE was measured using medians and interquartile ranges (IQR). AWilcoxon Signed Ranks Test for significance was used to analyse SpO2 and hyperoxia vs normoxic blood lactate data, due to remaining outside normality. HR was analysed under a paired sample t-test, to identify significance.Probability of significance for the study was set at p < 0.05. Tables and percentages were produced within SPSS to display these variables.
Results
Figure 1 presents pre and post blood lactate concentrations for both the hyperoxia and normoxic visits. During the portable O2 visit (Visit 2) blood lactate had risen by 24% post cycling (pre = 1.34 ± 0.29 mmol/L; post = 1.66 ± 0.26 mmol/L), showing a non-significance (p > 0.27). When compared to the normoxic visit (Visit 3), blood lactate had increased by 89% from the same exercise test (pre = 1.13 ± 0.23 mmol/L; post = 2.14 ± 0.24 mmol/L). This demonstrated a significantly different blood lactate concentration between pre and post cycling during normoxia (p < 0.01). Further significance (p < 0.04) was witnessed when comparing both post hyperoxia and normoxic visits for blood lactate concentrations, which found blood lactate concentrations were 29% lower during the portable recreational O2visit (Visit 2) compared to breathing regular air (Visit 3). Pre-cycling during the normoxic visit (Visit 3) yielded 15% more blood lactate within our sample when compared to the hyperoxia visit (Visit 2), which was non-significant (p > 0.06).
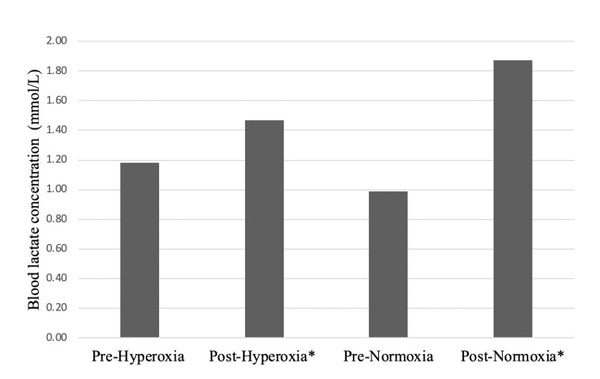
* = Significant (p < 0.05).
Figure 1 displays mean blood lactate concentration(mmol/L) during the pre and post hyperoxia and normoxic visits
Within the other parameters tested, a non-significance was concluded (p > 0.05) for HR, SpO2and RPE with mean (±SD) displayed in Table 3. No significance was found between hyperoxia and normoxic for HR (p > 0.73). A non-parametric testing on SpO2 between hyperoxia and normoxic was found to be non-significant (p > 0.19). RPE through Borg’s 6-20 scale withheld a non-significant finding between both visits (p > 0.25).
Table 3 displays the average of each of the five (5) efforts of exercise, established into mean± SD of HR and SpO2, and median, IQR of RPE for each participant (n=7) during the hyperoxic vs normoxic
|
Physiological data |
Hyperoxia vs Normoxia |
|
HR |
96 ± 5.9 vs 99 ± 4.2 |
|
SpO2 |
97 ± 0.2 vs 98 ± 0.2 |
|
*RPE |
11, 4 vs 12, 3 |
*RPE: Shown as medium, IQR
Discussion
The purpose of this study was to determine whetherinhaling a portable O2 supplement, as followed by the recreational O2 manufacturer’s guidelines, produces physiological benefits during moderate intensity cycling. The main findings of this study reveal that blood lactate remained significantly lower (p < 0.04), yielding a 29% decrease after moderate intensity cycling when using portable O2, compared to inhaling normal air. This is the first study to find a significant result whilst supplementing with this form of O2 inhalation and confirms some physiological benefit when the product is used with exercise. Stellingwerff et al. (2005) found similar significant findings when using O2tanks, concluding that inhalation of O2 at an FiO2= 60% for 20 minutes prior and 15 minutes during cycling at a moderate intensity decreasedblood lactate accumulation by 29% when compared to their normoxic visit. Continuing work by Stellingwerff et al. (2006) found cycling for 0 to 10 minutes at 70% V̇O2peak found a decrease in the accumulation ofblood lactate whilst inhaling O2 at FiO2 = 60%. In sum, these studies signify the finding that shorter exercise durations, have a substantial decrease toblood lactate when hyperoxia is administered.
Additional to our findings, there is a plethora of previous literature on the physiological reasoning for the decrease inblood lactate while supplementing with O2during exercise. Yokoi et al. (2014) speculate that the decrease inblood lactate could be attributed to a slower net breakdown of glycogen during exercise. Coincidently, Stellingwerff et al. (2005) detailed this slower carbohydrate utilization vs. oxidation during hyperoxia administration. This may have been the reasoning for our decrease inblood lactate, with increased amounts of O2 during moderate exercise promotingblood lactate metabolism over a prolonged resting state (Bangsbo et al., 1994). More evidence by Hauser et al. (2014) concluded thatblood lactate concentrations were lower after three, 3-minute double sprints efforts when hyperoxia was administered during recovery. They reported that due to the elevated oxidative capacity of working muscles, endurance-based exercise could benefit greatly from the enhanced clearance ofblood lactate during hyperoxic recovery after training. Therefore, in support of previous literature and our findings, these portable O2supplements could yield endurance-prolonging properties during moderate intensity cycling and other endurance-based activities, such as running, rowing or swimming.
Further physiological consideration for portable O2supplementation can be highlighted during the initiation of exercise. From rest, exercise increases pulmonary ventilation through a combination of tidal volume and respiratory rate to closely replicate the increase in V̇O2and the removal of CO2. As physical activity increases, the cardiorespiratory system begins to limit an individual’s maximal potential (Bassett & Howley, 2000). To overcome this, hyperoxia has shown to increase the capability in delivering O2to muscle cells and mitochondria, as V̇O2 is limited by the maximal capacity of the cardiorespiratory system in conjunction with the heart (Burton, Stokes & Hall, 2004). This was witnessed by Kim and Greenburg (2013) during maximal effort exercise limiting the cardiorespiratory system of their sample, which was not able to effectively deliver or utilise the O2 at a higher metabolic rate for ATP production. Bassett and Howley (2000)state that only trained individuals and athletes are prone to undergo arterial O2desaturation during maximal work, as they have a much higher maximal cardiac output, leading to a decrease pulmonary capillary. Therefore, smaller or less conditioned muscle groups may be limited by exercise and result in lower metabolic performance outcomes (Sperlich et al., 2011). The moderate intensity cycling established in our study could have resulted in cardiovascular principles not being stressed enough to warrant an issue in the amount of O2capillarisation from exercise. Further research to revisit under maximal intensity exercise with portable recreational O2 applied under the correct manner could yield positive results where Etheredge, Judge and Bellar (2014) did not.
Reasoning on why our study may have yielded positive results, where previous studies may have not, may be attributable to the type of duration and intensity used. Sperlich, et al. (2011) and Yokoi et al. (2014) highlighted that exercise performed maximally or self-paced yielded no differences in reported blood lactate levels between normoxic and hyperoxia visits. With this, hyperoxia does not appear to provide the same beneficial effect on energy utilisation during self-paced or maximal intensity exercise under a short duration. Segizbaeva and Aleksandrova (2009) witnessed this, concluding hyperoxia increases performance whilst decreasing the development of muscles fatigue evoked by incremental mild resistive cycling, but fails to yield similar effects during heavier loads. However, as Yokoi et al. (2014) concluded from previous literature by Maeda and Yasukouchi (2001), which mirror our findings, exercise performed under a fixed workload reducesblood lactate concentration in a hyperoxia environment. Thus, exercise intensity in a moderate effort could have been the reasoning for a lack of results within our remaining parameters (HR, RPE & SpO2), where other studies found significance.
Limitations and further direction
Limitations found in our study, as well as previous literature, relate to the amount of FiO2required to witness physiological benefits. Previous studies (Mallette et al., 2017) highlight that the majority of FiO2 used to promote physiological effects during physical activity is ≥60%. However, no research has been conducted comparing differing percentages of FiO2and their physical effects during exercise. Although, there is a plethora of studies that have confirmed the negative effects of participants when exposed within hypoxic environments (<21% FiO2) during exercise (Linnarsson et al., 1974; Zhang & Vincent, 1993; Rice et al., 1999; Brinkmann et al., 2018). Therefore, the logistics and practical application of portable recreational O2 canisters are cause for further evaluation. With no clear scientific quantity to administer to participants for beneficial physiological effect, in conjunction with previous literature, this study used a quantity deemed applicable for general use of supplementation. To date, no study has measured the number of breaths, frequency of inhalation and/or the duration of inhalation – measurements that would adequately initiate physiological benefits from hyperoxia, nor to what extent these benefits could enhance physiological principles during exercise. Further research with this perspective couldrevisit portable recreationalO2 supplements under moderate intensity exercise, witnessing if a greater effect to physical endurance properties exists. Maximal intensity exercise could also be revisited with the correct application of the O2 supplement, witnessing whether positive effects are applied similarly to other hyperoxic maximal intensity exercise studies.
Conclusion
From our findings, Oxygen Plus, Inc.’s portable recreational O2 supplement did influence recovery principles during fixed, short-term exercise. Administering this portable recreational O2supplement prior, during and post moderate intensity cycling, as well as other similar athletic activity, can yield positive results by significantly decreasingblood lactate concentrations; enabling individuals to increase endurance and speed of recovery time.
Acknowledgement
The portable recreational O2 supplements for this study were supplied by Oxygen Plus, Inc. Minnesota, USA. The author thanks Oxygen Plus, Inc. for willingly allowing it to be used within this study. We also thank the Sport and Exercise department at the University of Chichester for allowing us to undertake the study within their facilities. Lastly, we thank the participants who volunteered in this research, without whose efforts this study would not have been possible.
References
